ADVERTISEMENT
Mechanical Deviation of the Esophagus During Atrial Fibrillation Ablation: The Esophageal Retractor and More
Abstract
Catheter ablation of atrial fibrillation (AF) is more widely available and considered now to be the most performed catheter ablation procedure. Permanent electrical isolation of the pulmonary veins (PV) is well established as the cornerstone of AF ablation. Despite the importance of this ablation endpoint, permanent electrical isolation of the PVs may not be achieved in all patients. By its nature, this procedure involves ablation and catheter manipulation in the thin-walled atria, which are close to other important structures and organs. Esophageal injury is one of the most important complications associated with catheter ablation of AF. Mechanical deflection of the esophagus away from the ablation area may enable more effective RF lesion delivery while avoiding esophageal thermal damage. This review aims to summarize the current evidence for the EsoSure Esophageal Retractor (EPreward, Inc.) and provide information for its safety and efficacy, and review the study that examined its use in patients undergoing RF ablation in AF.
KEY WORDS: atrial fibrillation, esophageal injury, esophageal retraction device, radiofrequency catheter ablation
Introduction
Despite good progress in the management of patients with AF, this arrhythmia remains one of the major causes of stroke, heart failure, sudden death, and cardiovascular morbidity in the world.1
During the past few decades, surgical and radiofrequency (RF) catheter ablation of AF has evolved from investigational procedures to their current role as effective treatment options for patients with AF.2 Radiofrequency catheter ablation of AF includes electrical isolation of the pulmonary veins and may need additional ablations in the left atrial posterior wall, left atrial appendage, superior vena cava, and tricuspid isthmus to eliminate the foci causing AF. It has become the standard therapy for drug-refractory symptomatic AF. The extent of RF ablation varies from ablation of the pulmonary veins to complex and extensive ablation in the left atrium, generally involving the entire posterior wall.1,3,4 Although the procedure is safe, the significant anatomical variability in the relationship between the left atrium (LA) and the esophagus places the esophagus in a vulnerable position that is prone to thermal injury. Different variables affecting heat transfer to the esophagus have been identified in recent years, and new strategies to minimize the risk of esophageal injury are now used; however, they have not eliminated risks.3 The most serious and feared complications of this procedure are thermal esophageal injury, atrial-esophageal fistulation (AEF), and mediastinitis.5,6 Those complications may be associated with high morbidity and mortality.7 A rise in esophageal luminal temperature during RF ablation has been demonstrated as a marker for thermal injury.8 That is why luminal esophageal temperature monitoring during RF ablation is routinely performed in almost all centers in the world.9,10
A significant and rapid increase in luminal esophageal temperature during left atrial posterior wall ablation can cause injury in the esophageal tissue behind the LA wall. Shorter duration, lower power, and lower force RF ablations are generally used to decrease the damage to the esophagus. However, these RF applications may decrease the success of the procedure due to incomplete ablation.
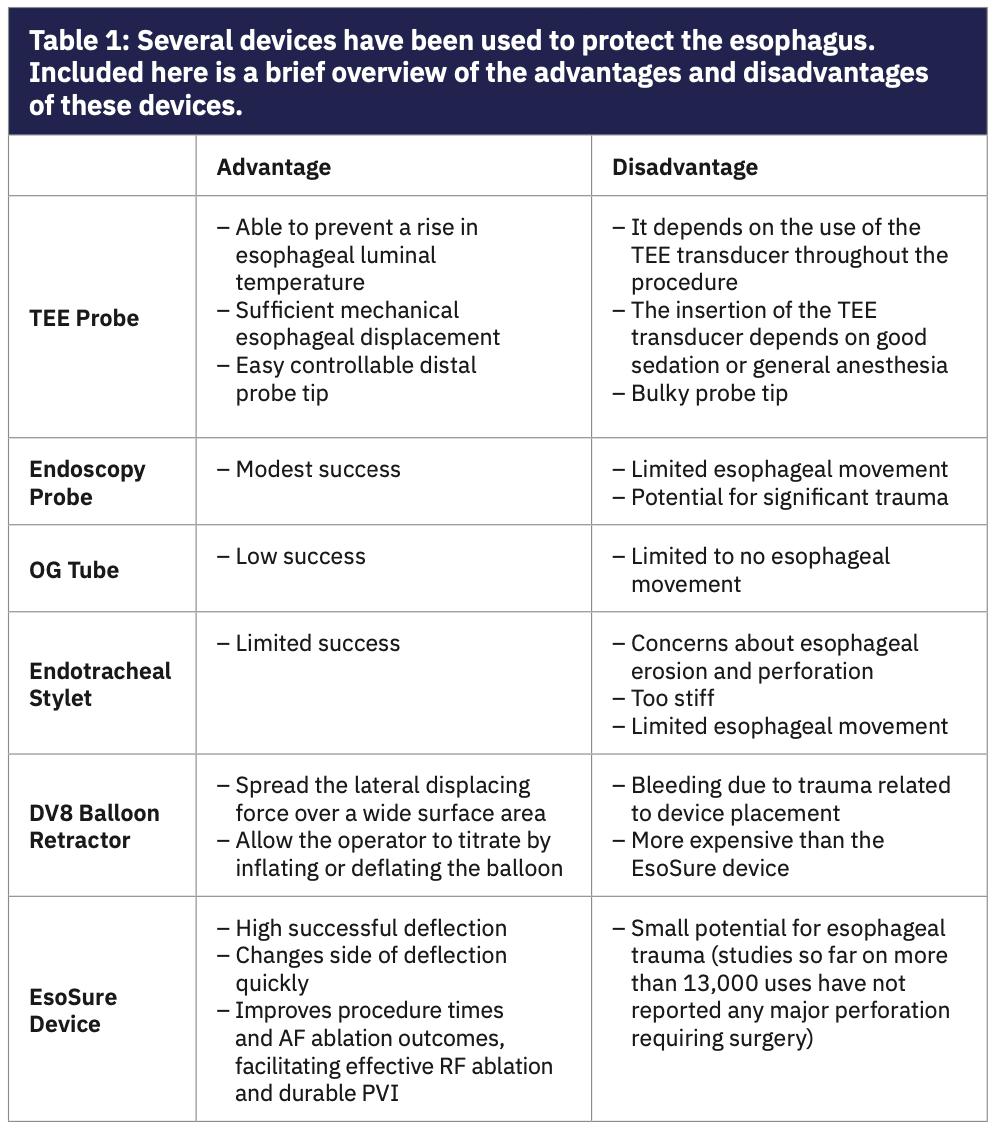
The mechanical deviation of the esophagus away from the ablation area may enable more effective RF lesion delivery while preventing esophageal thermal injury. Several techniques have been proposed to protect the esophagus (Table 1).11,12 The EsoSure Esophageal Retractor (EPreward, Inc.) has been improved for use for esophageal deviation. There is a lack of data regarding the EsoSure deflection device, limited to a single study.13 Therefore, we aimed to review the EsoSure device and its clinical use, to evaluate how it works in RF catheter ablation of atrial fibrillation.
Esophageal and Cardiac Anatomy
The esophagus, which is located at the center of the mediastinum, is separated from the LA by only a thin layer of fatty tissue and/or the pericardial sac and may be in contact with the left atrium. The relationship of the esophagus to the LA posterior wall and pulmonary veins is variable. The left atrium wall thickness is about 2-4 mm, and the esophageal thickness is 2-3 mm. The esophagus is supported at its upper end near the trachea and transits the diaphragm to connect with the stomach. The esophagus is supported at its lower end by the diaphragm as well. The thoracic portion of the esophagus between the trachea and the stomach is mobile and loosely restrained only by soft tissue. Behind the trachea, there are only loose membranes and sparse, thin muscle fibers connecting the upper esophagus to the trachea. The anatomy of the heart and esophagus is highlighted in Figure 1. There is no restrictive tissue attached to the esophagus below the carina. This allows the esophagus to move in response to swallowing food, cardiac and lung movement, and upper body movements.14 The esophagus is not a rigid, inflexible pipe but rather like a hose made of flexible muscle fibers. It can naturally migrate side to side 2-3 cm on its own.
Esophageal Injury During AF Ablation
AF ablation may cause collateral damage involving multiple structures neighboring the heart. Some immediately neighboring structures sustain a reasonably well-described direct thermal injury.4 However, many other structures probably sustain a combination of direct microvascular, thermal, and regional nervous system injuries. Some of the well-identified gastrointestinal system complications, including esophageal ulceration, gastroesophageal reflux, atrial-esophageal fistulae, gastric hypomotility, and acute pyloric spasm are at least partially a consequence of vagal nerve (VN) injury.4 The mechanisms of these injuries are probably multifactorial. The VN and its branches innervate most of the gastrointestinal system. The VN and its branches are anatomically closely related to the posterior wall of the LA and the PVs. The right and left VN comes down alongside the esophagus into the abdomen, and their course in the posterior mediastinum is variable.4 VN injury can be a result of a direct neuronal injury from RF ablation as evidenced by decreased gastric myoelectric impulses.4,15 It can also be a result of compression of plexus from edema resulting from an RF ablation-induced inflammatory response of the neighboring mediastinal structures (indirect effect of RF ablation). Microhematomas from vascular injury during RF ablation may also cause the same effect on this plexus.4
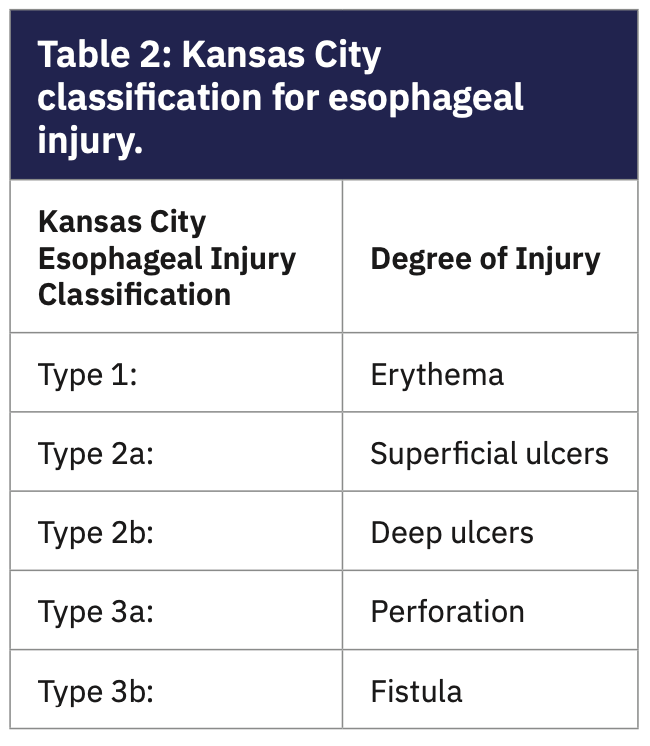
Injury to the vagal anterior esophageal plexus can occur when RF energy is applied to the posterior wall of the LA, which can cause acute pyloric spasm and gastric hypomotility. Atrio-esophageal fistula is the most devastating complication, with an incidence of .001 to .003 and >50% fatality. Patients can suffer various degrees of esophageal injury due to the indirect effect of ablation; esophageal lesions can be identified using the novel Kansas City classification (KCC) (Table 2 and Figure 2).16
Factors Impacting Atrio-Esophageal Injury
Factors that impact atrio-esophageal injury include general anesthesia, elderly age, female gender, posterior wall ablation, lesion duration, catheter tip size, use of contact force, and RF ablation power.
Other factors, such as the energy delivered to the LA posterior wall, additional ablation lines, LA size, esophageal-LA distance, use of a nasogastric tube in general anesthesia, and chronicity of AF, have been identified as predictors of esophageal complications.17 The maximal esophageal temperature has been demonstrated to be significantly higher in patients undergoing general anesthesia versus patients undergoing conscious sedation. Lower pressure levels might decrease the incidence of esophageal injury but also prevent transmurality of the lesions, and therefore, increase the risk for AF recurrence.18,19 Due to increased catheter stability, energy delivery using a remote robotic navigation system is more efficient, resulting in deeper lesions and thus bearing the potential of increased esophageal damage at ablation sites close to the esophagus.20 Strategies used to prevent esophageal injury were reducing power to 25 W, reducing ablation duration to 30 seconds in the posterior wall, and ICE guidance to restrict microbubble formation.17 Suggestions, originally from surgical literature, recommend the reduction of power for lesions along the posterior wall of the LA.21 On the other hand, it is possible that a more conservative temperature setting may result in less effective lesions and a higher recurrence rate.21 Annual operator and hospital volume have emerged as an important factor predicting adverse outcomes.22
Esophageal Temperature Monitoring
During the AF ablation procedure, luminal esophageal temperature measurement and follow-up and modification of the ablation lesion area and energy delivery settings became standard practice. Unfortunately, because the esophagus is broad, the lateral position of the temperature probe or mapping electrode might not align with the ablation electrode, and the operator could receive a false impression of safety.2 Many institutions use an esophageal temperature probe to prevent thermal injury; however, it is widely acknowledged that the use of an esophageal temperature probe does not eliminate the risk of esophageal injury.2,23 Often, the ablation procedure is stopped after luminal esophageal temperature rise to allow the esophagus to cool down. This may cause suboptimal results and increase arrhythmia recurrence.
Methods of Preventing Esophageal Injury
Aggressive monitoring. Although the optimal technique for avoiding esophageal injury has not yet been determined, the use of a temperature probe is encouraged in the current guidelines for all patients.20 Moreover, recently published data have shown that the use of an esophageal temperature probe might be associated with a significantly lower incidence for esophageal injury when compared with patients having undergone pulmonary vein isolation with the same energy settings but without temperature monitoring.20 But routine use of a temperature probe is not an effective method to prevent esophageal injury, because it does not reflect the exact temperature in the esophagus.
Esophageal cooling. The cooling methodology consisted of the infusion of a small volume of cold liquid at a time. Meta-analysis studies have found that even with the limited heat extraction possible using this method, there was a reduction in the number or severity of lesions.24
Using lower power on the posterior wall. To minimize injury to the esophagus during AF ablation applications on the posterior wall close to the esophagus, several approaches can be employed, including shortening RF application time (eg, <20 seconds), reduction of radiofrequency power (eg, <25 W), and/or decreasing contact force (eg, <10 grams).
High power and short duration. Often, RF ablation is halted after luminal esophageal temperature rise to allow the esophagus to cool down. Repeated on-off ablation could lead to suboptimal lesion formation and an increase in AF recurrence.4 Although its efficacy has been established, catheter ablation of AF is associated with a substantial risk of AF recurrence. High power short duration (HPSD) ablation is becoming increasingly popular to mitigate collateral injury to the esophagus. The early clinical data is promising; however, a large data set is yet to be generated to appreciate the true impact of the esophageal injury, especially AE fistula. It is important to recognize that late recurrences are often asymptomatic. Recurrences of AF after ablation are generally classified into 3 types according to the phase after ablation in which they appear: (1) early recurrence (within 3 months); (2) late recurrence (from 3 months to 1 year); and (3) very late recurrence (more than 1 year). The characteristics and optimal managements differ according to the type of recurrence. Patients with later recurrences were more likely to have sporadic episodes and respond better to antiarrhythmic medications and repeat ablation. This observation suggests pathophysiological differences based on time to recurrence and has implications for clinical management.2
Mechanical deviation of the esophagus. Various mechanical esophageal deviation devices such as the endoscopy probe, transesophageal echocardiography probe, endotracheal stylet, DV8 Retractor (Manual Surgical Sciences), and EsoSure have been used. The endoscopy probe, transesophageal echocardiography probe, and endotracheal stylet placed in the thoracic chest tube have been tried with modest success.11,12,25 However, their routine use has been limited because of concerns about potential esophageal injury.
Cryoballoon ablation. Cryoballoon ablation has emerged as an alternative technique to RF ablation for the treatment of patients with symptomatic AF within the past 10 years. Cryoballoon ablation is known to be similarly effective to RF ablation; however, the risk of cryo-related esophageal injury and AE fistula has not gone away.2
Pulsed field ablation (PFA). Various implementations of PFA are possible and applicable to future implementations of PFA using different catheter technologies and waveform compositions. Importantly, if the magnitude of the pulse amplitude is high enough, the tissue-selective properties of PFA would likely be lost and indiscriminate tissue necrosis could ensue. It has been demonstrated that in patients with paroxysmal atrial fibrillation, PFA rapidly and efficiently isolates PVs with a degree of tissue selectivity and a safety profile heretofore not described for cardiac ablation.26
Why Mechanical Deviation of the Esophagus Works
The esophagus is surrounded by soft tissue and is a mobile organ. It can be manipulated easily with mechanical deviation devices. Esophageal position can be changed at any time during RA ablation. Besides during ablation on the right side, the esophagus can be moved to the other side, and the reverse can be done. The EsoSure device can move the esophagus in every direction without any limitation.
The EsoSure Esophageal Retractor
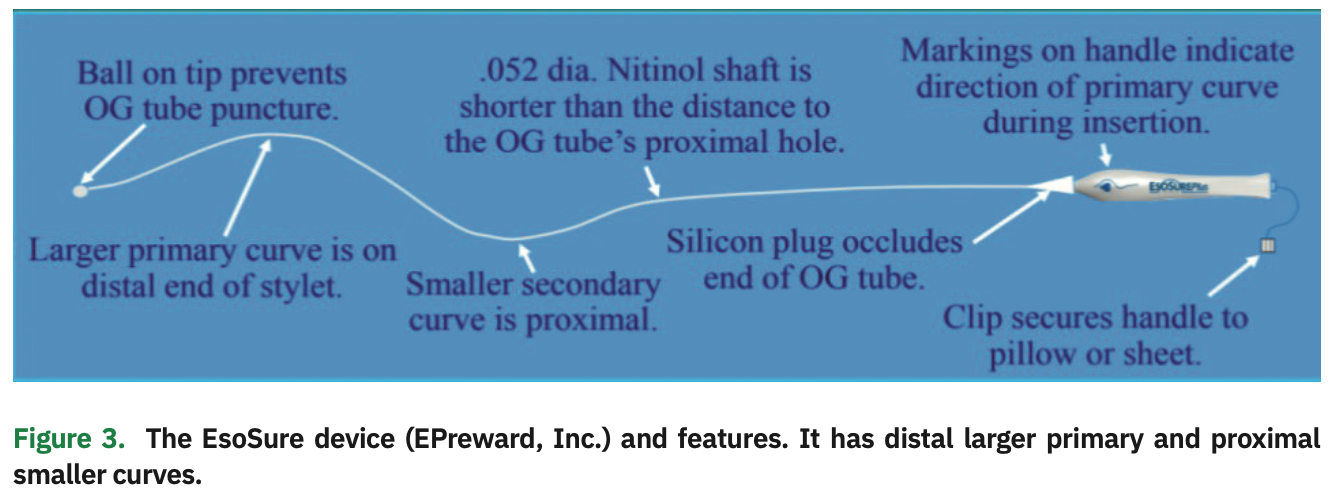
The EsoSure device is a .052-inch diameter, temperature-programmed Nitinol stylet that is flexible at room temperature for placement into the lumen of an 18 Fr orogastric (OG) tube and assumes a firm S-shaped curve at body temperature to create esophageal deflection (Figure 3). At room temperature, the stylet is fairly straight, allowing it to be easily placed into a commonly used OG tube placed in the esophagus and stomach. As the stylet warms to body temperature, it takes on a greater distal curve. Depending on how the stylet is positioned, it can displace the esophagus up to 1-5 cm to the right or left, depending on each person’s esophagus anatomy.
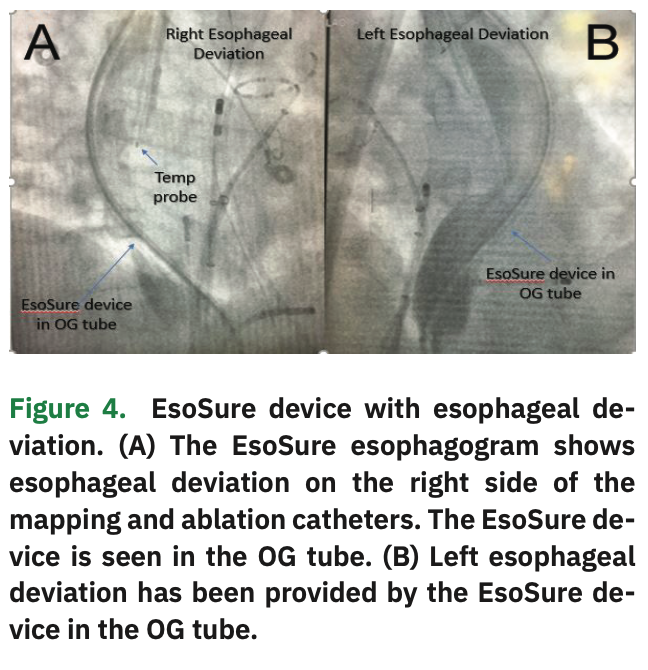
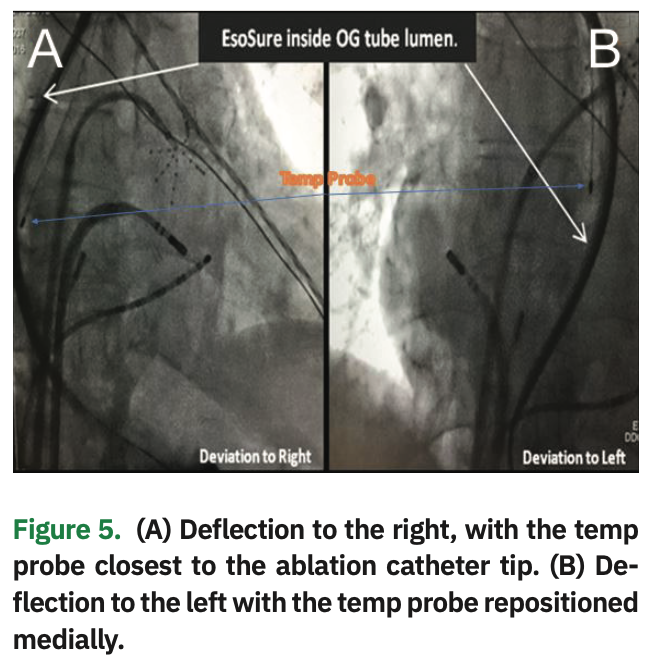
The device allows physicians to relocate a section of the esophagus away from the adjacent heart tissue and avoid the heat generated during radiofrequency ablation (Figure 4). Using the EsoSure device, the EP staff can safely and easily move the esophagus away from any region being ablated. Simply move the EsoSure Retractor down and up to displace the esophagus to ablate in the posterior of the LA (Figure 5).
The goals and potential benefits of moving the esophagus, as described in the literature, include minimizing thermal damage to the esophagus, allowing for the placement of appropriately deep ablation, reducing procedure time by decreasing the frequency, and increasing procedural success rates.4
The DEFLECT GUT Study
In the multicenter DEFLECT GUT Study, Parikh et al evaluated the safety and efficacy of the EsoSure device in 687 patients who underwent RF ablation for AF (209 patients in the EsoSure group, and 478 patients in the control group).13 Esophageal deviation was used for left-sided PVs in 133 patients (74%), right-sided PVs in 38 patients (21%), and the posterior wall in 9 patients (5%); however, there was no statistical difference in the location of the esophagus between all groups. Esophageal temperature rise >1oC and total procedural time significantly increased in the non-EsoSure group (79.4% vs 3%; P<.001). The non-EsoSure group had a higher luminal esophageal temperature rise (1.66±.54 vs .34±.59; P<.001) than the EsoSure group. Although the total fluoroscopy time was found to be the same in both groups, the total procedure time was significantly shorter in the EsoSure group than the non-EsoSure group (182±36 minutes vs 206±32 minutes; P<.001). Also, acute pulmonary vein isolation was found to be the same between the 2 groups. AF recurrence rates (at 3, 6, and 12 months) were lower in the EsoSure group (P<.5).13
This study determined that displacement of the esophagus with the EsoSure device from the area of ablation was safe for enabling effective lesion delivery without a significant increase in luminal esophageal temperature as well as a decreased risk of esophageal injury. However, they did not perform a routine post-procedural endoscopy to evaluate for possible esophageal lesions with device use. There was no difference in overall complications between groups (3.8% vs 3.8%; P=1).13 The study also showed that the mechanical displacement of the esophagus using the EsoSure decreases procedure time and improves short- and long-term AF outcomes. In this study, major and minor complications of the procedure in the EsoSure group occurred in 7 patients (3.8%). Two patients developed pericardial effusion: 1 did not require intervention, and the other needed cardiac surgery. No patients experienced a transient ischemic attack, stroke, or death.
EsoSure Insertion and Positioning: Step-by-Step Instructions
Before insertion: Ask the patient about contraindications, esophageal abnormalities.
1: Endotracheal intubation is required for Eso-Sure use;
2: Insert single or multi-pole temp probe with extra lubrication;
3: Insert 18 Fr 48” Salem Sump OG tube before anticoagulation;
4: Inject contrast behind left atrium;
5: Reposition OG tip into the stomach and flush with 8-10 cc of lubricant (fluid, gel, or olive oil).
Insertion of the EsoSure:
– Use the coronary sinus as the floor of the LA;
– If deflection is poor, use the ventilator to change the anatomy and improve deflection;
– Use propofol or sedatives to relax the airway and avoid a gag reflex;
– Open the airway, by a second person, with a head tilt-jaw lift for easier and safer stylet insertion;
– Cool OG tube to slow stylet’s curving by injecting 20 cc room temp IV fluid/water;
– Hold an OG tube up 45° straight from the mouth;
– Advance EsoSure immediately into OG tube lumen. Watch with fluoro past the throat. Advance OG tube and EsoSure together if tip stops above CS. Stop when stylet tip is past the CS or primary curve is at the upper heart (Figure 6);
– Rotate the primary curve to the desired side with the primary curve behind the heart. Then slide the primary curve back down behind the heart;
– Initiate Valsalva if the esophagus does not cross mid-spine. Rotate the primary curve to the desired side behind the trachea, perform a Valsalva, then advance the curve behind the heart;
– Temp probe is positioned at the same level as the maximum EsoSure curve;
– Assess the esophageal trailing edge with fluoro or contrast.
Ways to change from right deflection to left deflection:
– Advance EsoSure so secondary curve deflects to the left;
– Advance the temp probe into the stomach;
– Advance OGT/EsoSure for L deflection with a secondary curve;
– Retract temp probe;
– When the primary curve is R below the CS/diaphragm, do not rotate EsoSure.
How to rotate the primary curve from right to left:
– Advance temperature probe;
– Retract the EsoSure from the OG tube so the tip is above the diaphragm;
– Rotate the handle 2-3 times, then slowly retract the handle out of OGT. Watch it with fluoro; if it spins, rotate in the opposite direction;
– After rotation to the correct side, release torque and advance curve to the desired position;
– Retract temp probe;
– When the primary curve is to the left, do not advance EsoSure past the diaphragm.
Cost Considerations
The EsoSure Esophageal Retractor Device is priced at $365 to $395. The device is relatively easy to use and can easily deviate the esophagus to allow successful posterior wall ablation. This can improve the short- and long-term recurrences of AF, and will reduce the need for redo ablations and the potentially devastating complications of esophageal injury. All these factors translate into indirect and direct cost savings. There is a learning curve, as usual; the learning curve, as with many other skills learned in the EP lab, varies from 5 to 15 uses and depends on the new user’s frequency of use, knowledge of fluoro images, manual experience with handling wires and tubes, and self-confidence in learning new skills.
Conclusion
There are several benefits with the EsoSure Esophageal Retractor Device. It can be inserted into the lumen of a standard OG tube in 5 seconds, achieve successful deflection in >80% of cases (range of .8 cm to 5 cm), change the side of deflection in seconds, and mimic natural migration. There is no esophageal injury caused by the EsoSure device.
Temporary mechanical changing of the esophagus’s shape with the EsoSure device seems to be effective and safe during isolation of the pulmonary veins and LA posterior wall without significant intraluminal temperature increase in the esophagus. These interrelations need to be clarified in further prospective randomized studies with post-procedural endoscopy for any esophageal lesion with the use of the EsoSure device.
Disclosures: Dr. Elbey, Dr. Kanuri, Ms. Atkins, Dr. Gopinathannair, Dr. Swarup, Dr. Oza, and Dr. Della Rocca have no conflicts of interest to report regarding the content herein. Dr. Daccarett reports he is a consultant for Boston Scientific. Steve Miller is the founder and President of EPreward, and inventor, R&D lead, distributor and trainer for the EsoSure Esophageal Retractor; he reports that he provided his research and training documents to the authors to be used at their discretion in the writing of the article. Dr. Di Biase is a consultant for Biosense Webster, Boston Scientific, and Abbott (formerly St. Jude Medical), and has received speaker honoraria/travel from Medtronic, AtriCure, Pfizer, Bristol-Myers Squibb, and BIOTRONIK. Dr. Natale reports personal fees as a consultant from Abbott, BIOTRONIK, Boston Scientific, Baylis Medical, Biosense Webster, and Medtronic. Dr. Lakkireddy reports personal fees as a consultant from Abbott, Boston Scientific, BIOTRONIK, and Medtronic.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-76.
- Lakkireddy D, Reddy YM, Atkins D, et al. Effect of atrial fibrillation ablation on gastric motility: The Atrial Fibrillation Gut Study. Circ Arrhythm Electrophysiol. 2015;8:531-536.
- Dar T, Yarlagadda B, Alkhatib C, Lakkireddy D. Esophageal laceration related to mechanical trauma from a general purpose (esophageal/rectal) temperature probe introducer sheath during atrial fibrillation ablation. J Atr Fibrillation. 2018;10(5):1878.
- Katz-Agranov N, Nevah Rubin MI. Severe esophageal injury after radiofrequency ablation – a deadly complication. World J Gastroenterol. 2017;23(18):3374-3378.
- Sonmez B, Demirsoy E, Yagan N, et al. A fatal complication due to radiofrequency ablation for atrial fibrillation: atrio-esophageal fistula. Ann Thorac Surg. 2003;76:281-283.
- Kiuchi K, Okajima K, Shimane A, et al. Impact of esophageal temperature monitoring guided atrial fibrillation ablation on preventing asymptomatic excessive transmural injury. J Arrhythm. 2016;32:36-41.
- Koranne K, Basu-Ray I, Parikh V, et al. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation: a meta-analysis. J Atr Fibrillation. 2016;9:1452.
- Deneke T, Bunz K, Bastian A, et al. Utility of esophageal temperature monitoring during pulmonary vein isolation for atrial fibrillation using duty-cycled phased radiofrequency ablation. J Cardiovasc Electrophysiol. 2011;22:255-261.
- Mateos JC, Mateos EI, Pena TG, et al. Simplified method for esophagus protection during radiofrequency catheter ablation of atrial fibrillation — prospective study of 704 cases. Rev Bras Cir Cardiovasc. 2015;30:139-147.
- Koruth JS, Reddy VY, Miller MA, et al. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:147-154.
- Parikh V, Swarup V, Hantla J, et al. Feasibility, safety, and efficacy of a novel preshaped nitinol esophageal deviator to successfully deflect the esophagus and ablate left atrium without esophageal temperature rise during atrial fibrillation ablation: the DEFLECT GUT study. Heart Rhythm. 2018;15:1321-1327.
- Sivarao DV, Goyal RK. The DEFLECT Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000;108(Suppl 4a):27S-37S.
- Tilz RR, Chun KR, Metzner A, et al. Unexpected high incidence of esophageal injury following pulmonary vein isolation using robotic navigation. J Cardiovasc Electrophysiol. 2010;21:853-858.
- Yarlagadda B, Deneke T, Turagam M, et al. Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation ablation. Heart Rhythm. 2019;16(2):204-212.
- Bhaskaran A, Chik W, Thomas S, Kovoor P, Thiagalingam A. A review of the safety aspects of radio frequency ablation. Int J Cardiol Heart Vasc. 2015;8:147-153.
- Di Biase L, Natale A, Barrett C, et al. Relationship between catheter forces, lesion characteristics, ‘popping,’ and char formation: experience with robotic navigation system. J Cardiovasc Electrophysiol. 2009;20:436-440.
- Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724-2726.
- Rillig A, Meyerfeldt U, Birkemeyer R. Oesophageal temperature monitoring and incidence of oesophageal lesions after pulmonary vein isolation using a remote robotic navigation system. Europace. 2010;12:655-661.
- Cummings JE, Schweikert RA, Saliba WI, et al. Assessment of temperature, proximity, and course of the esophagus during radiofrequency ablation within the left atrium. Circulation. 2005;4(112):459-464.
- Deshmukh A, Patel NJ, Pant S, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010 analysis of 93 801 procedures. Circulation. 2013;128:2104-2112.
- Chugh A, Rubenstein J, Good E, et al. Mechanical displacement of the esophagus in patients undergoing left atrial ablation of atrial fibrillation. Heart Rhythm. 2009;6:319-322.
- Leung LW, Gallagher MM, Santangeli P, et al. Esophageal cooling for protection during left atrial ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2019. doi.org/10.1007/s10840-019-00661-5.
- Deneke T, Schade A, Krug J, et al. Predictors of recurrence after catheter ablation of persistent atrial fibrillation. J Atr Fibrillation. 2012;4(5):498.
- Reddy VY, Neuzil P, Koruth JS, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315-326.