ADVERTISEMENT
Initial Experience With the PentaRay NAV Catheter: Clinical and Operational Considerations
 The Arrhythmia Center at Indiana University Health La Porte Hospital recently utilized a new technology, Biosense Webster’s PentaRay NAV catheter, during an atrial tachycardia ablation. Drs. Mark Dixon and Scott Kaufman, both cardiac electrophysiologists at IU Health La Porte Hospital, were aware of the availability of this new catheter as a result of discussions with our Biosense Webster Regional Education Specialist, Chris Fillier. These physicians identified a patient in the practice that met the criteria for use and were interested in
The Arrhythmia Center at Indiana University Health La Porte Hospital recently utilized a new technology, Biosense Webster’s PentaRay NAV catheter, during an atrial tachycardia ablation. Drs. Mark Dixon and Scott Kaufman, both cardiac electrophysiologists at IU Health La Porte Hospital, were aware of the availability of this new catheter as a result of discussions with our Biosense Webster Regional Education Specialist, Chris Fillier. These physicians identified a patient in the practice that met the criteria for use and were interested in  utilizing the catheter during his ablation. However, as with any new tool, adjunct for therapy, process or procedure, there is always a need for an assessment of efficacy before formal integration into the care delivery system can occur. Multiple questions were posed to the medical staff and colleagues related to the intra-procedure evaluation of the catheter so an adequate assessment could be achieved: Did this new catheter impact patient quality? Was it cost effective? Were there any safety issues that would put the patient at risk? Were we
utilizing the catheter during his ablation. However, as with any new tool, adjunct for therapy, process or procedure, there is always a need for an assessment of efficacy before formal integration into the care delivery system can occur. Multiple questions were posed to the medical staff and colleagues related to the intra-procedure evaluation of the catheter so an adequate assessment could be achieved: Did this new catheter impact patient quality? Was it cost effective? Were there any safety issues that would put the patient at risk? Were we  more efficient with this or without it? Was it easy to use? All these questions would be evaluated during the case to see if this was a new product that we would add to our current supply listing. The following article outlines various operational considerations for this new product as well as includes a case study describing the clinical considerations that we encountered for this patient.
more efficient with this or without it? Was it easy to use? All these questions would be evaluated during the case to see if this was a new product that we would add to our current supply listing. The following article outlines various operational considerations for this new product as well as includes a case study describing the clinical considerations that we encountered for this patient.
Operational Considerations
Software
 Although we were running the Carto 3 V2.3 software, an additional software upgrade (V3.1.1) was necessary for utilization with the multi-electrode mapping (MEM) technology from Biosense Webster. The upgrade was accomplished by Biosense Webster personnel in a short period of time.
Although we were running the Carto 3 V2.3 software, an additional software upgrade (V3.1.1) was necessary for utilization with the multi-electrode mapping (MEM) technology from Biosense Webster. The upgrade was accomplished by Biosense Webster personnel in a short period of time.
An additional advantage (not related to the new software) was gained during the upgrade. We found that we were not backing up our files on a routine basis, so a new policy 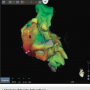 was implemented for the department and with Biosense Webster. This policy requires that at the end of each case, a CD-ROM is generated with the study on it and filed in the patient’s clinic chart for future reference. This alleviates sporadic, time-consuming backups and allows the system to run more efficiently.
was implemented for the department and with Biosense Webster. This policy requires that at the end of each case, a CD-ROM is generated with the study on it and filed in the patient’s clinic chart for future reference. This alleviates sporadic, time-consuming backups and allows the system to run more efficiently.
Catheter Characteristics
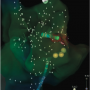
 The PentaRay NAV is recommended for use in cases of atrial tachycardia, ventricular tachycardia, and complex fractionated electrograms in atrial fibrillation. The intent of this catheter is to provide high-resolution maps with the fewest moves necessary to create them. It has a five-branched star design, and these branches are soft and very flexible in order to decrease risk of traumatic injury to the endocardium. Signal resolution has also been improved. In addition, pacing can be performed from any electrode.
The PentaRay NAV is recommended for use in cases of atrial tachycardia, ventricular tachycardia, and complex fractionated electrograms in atrial fibrillation. The intent of this catheter is to provide high-resolution maps with the fewest moves necessary to create them. It has a five-branched star design, and these branches are soft and very flexible in order to decrease risk of traumatic injury to the endocardium. Signal resolution has also been improved. In addition, pacing can be performed from any electrode.
 There are currently four PentaRay NAV catheters available for use. The catheters are available in both F and D curves. The shaft is a 7 French (Fr) and requires an 8 Fr sheath with a saline flush for the inner lumen of the sheath. Spacing consists of 4-4-4 and 2-6-2. There are 20 poles on each catheter divided among the five branches. The required interface cable to the PIU was already in our stock, as the PentaRay NAV utilizes the same cable as the original Lasso catheter (not the ECO). The C-Code for this catheter is C1732.
There are currently four PentaRay NAV catheters available for use. The catheters are available in both F and D curves. The shaft is a 7 French (Fr) and requires an 8 Fr sheath with a saline flush for the inner lumen of the sheath. Spacing consists of 4-4-4 and 2-6-2. There are 20 poles on each catheter divided among the five branches. The required interface cable to the PIU was already in our stock, as the PentaRay NAV utilizes the same cable as the original Lasso catheter (not the ECO). The C-Code for this catheter is C1732.
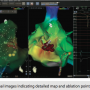 At this time, there are no recommendations as to which catheter spacing is best for certain patient conditions. Utilization depends on physician preference. We chose to utilize the D Curve catheter with the 4-4-4 spacing. However, it should be noted that although there are 20 poles on the catheter, it is not linear like a 10- or 20-pole catheter. Not every pole produces an intracardiac signal. The catheter with the 4-4-4 spacing has 15 intracardiac signals for viewing, while the 2-6-2 spacing has 10.
At this time, there are no recommendations as to which catheter spacing is best for certain patient conditions. Utilization depends on physician preference. We chose to utilize the D Curve catheter with the 4-4-4 spacing. However, it should be noted that although there are 20 poles on the catheter, it is not linear like a 10- or 20-pole catheter. Not every pole produces an intracardiac signal. The catheter with the 4-4-4 spacing has 15 intracardiac signals for viewing, while the 2-6-2 spacing has 10.
CardioLab Configuration
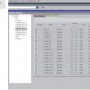 Configuration of the CardioLab was also an easy task after we thought through the process. GE’s CardioLab (software release 6.8.1) with a 128-channel amplifier is utilized in our main procedural lab. A study type is currently configured for Carto 3 mapping, which incorporates HRA, RVA, His, coronary sinus, Lasso and ablating catheters. In this study configuration, catheter input module (CIM; also referred to as Block A) was utilized for the HRA, His, RVA (for non-Biosense Webster catheters), and the stimulator connection to Carto 3. Block B was utilized for the ablation catheter. Block C was utilized for the coronary sinus and Lasso catheters. Blocks B and C are the interface between the CardioLab amplifier and the Carto 3 PIU.
Configuration of the CardioLab was also an easy task after we thought through the process. GE’s CardioLab (software release 6.8.1) with a 128-channel amplifier is utilized in our main procedural lab. A study type is currently configured for Carto 3 mapping, which incorporates HRA, RVA, His, coronary sinus, Lasso and ablating catheters. In this study configuration, catheter input module (CIM; also referred to as Block A) was utilized for the HRA, His, RVA (for non-Biosense Webster catheters), and the stimulator connection to Carto 3. Block B was utilized for the ablation catheter. Block C was utilized for the coronary sinus and Lasso catheters. Blocks B and C are the interface between the CardioLab amplifier and the Carto 3 PIU.
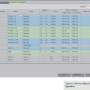 This original study was copied and re-titled “Penta” so all colleagues would be able to distinguish between the two Carto studies. However, as we started the input process, we quickly discovered that there would not be an adequate number of channels in the current configuration and it wouldn’t be as simple as renaming existing channels. Stim 1, 2, 3 and 4 channels are hardwired in the system and cannot be removed or shifted from their current positions on Blocks A and B. So, alternative positions would have to be found. In order to accommodate all necessary channels, the Carto 3 PIU was connected to Blocks B and D on the amplifier. If the catheter with the 2-6-2 spacing is utilized, colleagues choose Block B. If the catheter with the 4-4-4 spacing is utilized, colleagues choose Block D.
This original study was copied and re-titled “Penta” so all colleagues would be able to distinguish between the two Carto studies. However, as we started the input process, we quickly discovered that there would not be an adequate number of channels in the current configuration and it wouldn’t be as simple as renaming existing channels. Stim 1, 2, 3 and 4 channels are hardwired in the system and cannot be removed or shifted from their current positions on Blocks A and B. So, alternative positions would have to be found. In order to accommodate all necessary channels, the Carto 3 PIU was connected to Blocks B and D on the amplifier. If the catheter with the 2-6-2 spacing is utilized, colleagues choose Block B. If the catheter with the 4-4-4 spacing is utilized, colleagues choose Block D.
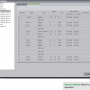 As discussed earlier, while the 4-4-4 spacing catheter has 20 poles, it is not linear, so there will not be 20 intracardiac signals for viewing. When configuring the PentaRay NAV 4-4-4 spacing, overlap is needed for the majority of poles. (See Figure 1 for Block D configuration.) This allows for 15 intracardiac signals for viewing and analysis. The ablating distal signal is displayed on this block; ablating proximal was not visualized during this study.
As discussed earlier, while the 4-4-4 spacing catheter has 20 poles, it is not linear, so there will not be 20 intracardiac signals for viewing. When configuring the PentaRay NAV 4-4-4 spacing, overlap is needed for the majority of poles. (See Figure 1 for Block D configuration.) This allows for 15 intracardiac signals for viewing and analysis. The ablating distal signal is displayed on this block; ablating proximal was not visualized during this study.
The PentaRay NAV 2-6-2 spacing is configured for Block B on the amplifier (see Figure 2 for Block B configuration). There is no overlap on this catheter, and 10 intracardiac signals for viewing and analysis are available. Stims 3 and 4 remain hardwired. In addition, the ablating distal and proximal signals are also configured on this block.
Block A contains HRA distal and proximal, RVA distal and proximal, His distal, mid and proximal, Carto Stim connections, and Stims 1 and 2. Because of the use of the PentaRay NAV catheter, an HRA catheter was not displayed while it was in use. (See Figure 3 for Block A configuration.)
Clinical Support
 A collaborative partnership had previously been established with Biosense Webster and the Arrhythmia Center. Clinical support for this case was provided by Regional Education Specialist Chris Fillier, who had an exceptional understanding of the product and facilitated the implementation of the new software and catheter use. Education was provided to the physicians and EP colleagues on site.
A collaborative partnership had previously been established with Biosense Webster and the Arrhythmia Center. Clinical support for this case was provided by Regional Education Specialist Chris Fillier, who had an exceptional understanding of the product and facilitated the implementation of the new software and catheter use. Education was provided to the physicians and EP colleagues on site.
Patient Preparation and Care
 Routine procedures were utilized for the preparation and care of this patient. Reference and ground patches were placed in the same format as other cases. Standard ECG electrode placement was utilized as well. There were no changes to the standard delivery of care in the pre-, intra-, or post-procedure periods.
Routine procedures were utilized for the preparation and care of this patient. Reference and ground patches were placed in the same format as other cases. Standard ECG electrode placement was utilized as well. There were no changes to the standard delivery of care in the pre-, intra-, or post-procedure periods.
Case Study
The patient is a 65-year-old African American male who was initially referred for evaluation of palpitations and an irregular heartbeat. The patient had stated that he was told during a routine work physical 30 years ago that he had an irregular heartbeat. However, no further action had ever been taken.
More recently, the patient had been evaluated by both his primary care physician and a general cardiologist for dyspnea and unusual fatigue, as well as risk factors for CAD including type II diabetes mellitus. A full cardiac work-up was performed, and the results were as follows:
• Holter monitor – 48 hours. The predominant rhythm was sinus. However, over 50% of the beats were tachycardic. There were 271 PVCs, 30 couplets and one 3-beat run of NSVT. There was no atrial fibrillation, but there were frequent episodes of atrial arrhythmias including over 1,100 isolated PACs, 1,300 couplets, and over 600 episodes of atrial bigeminy. The atrial arrhythmias were notable for almost 4,000 runs of SVT. The longest was 800 beats in duration at a rate of 140 bpm, and the fastest was at 170 bpm. It was interpreted by the general cardiologist as SVT, probably AVNRT. There was a 1.8-second pause occurring at the end of the supraventricular event. The patient did not report any symptoms on the accompanying patient diary.
• Echo, 2DM-Mode. LVEF 65% and mild dilatation of the right and left atrium as well as the right ventricle were noted. There was normal left ventricular size. No valve issues or effusions were seen.
• HVI Cardiolite Stress Test. During exercise, the patient’s EKG (completed stage II of the Bruce protocol and achieved a workload of 7.0 METS) transitioned to atrial fibrillation with a rapid ventricular response reaching a rate of 214 bpm. Diffuse nonspecific ST-T wave abnormalities were noted. Exercise-related dyspnea and fatigue were present. The imaging portion of the study indicated normal myocardial perfusion with no reversible defects suggestive of ongoing ischemia.
Appropriate referral was made to the Arrhythmia Center at IU Health La Porte Hospital. Dr. Kaufman performed the initial consultation on this patient. Current medications included metoprolol 25 mg bid, metformin 500 mg qid, Caduet 5/40 mg daily, Benicar HCT 20/12.5 mg daily, aspirin 325 mg daily, and Zyrtec 10 mg daily. No additional details were noted on the previously described history and diagnostic testing. Electrophysiologic testing with possible radiofrequency catheter ablation was recommended because of the patient’s paroxysmal supraventricular tachycardia in the setting of no structural heart disease as noted on echocardiogram. All risks and benefits were denoted during the consult. Both the patient and family agreed to the procedure as outlined by Dr. Kaufman. Routine pre-procedure instruction was given to the patient, including a three-day hold on metoprolol dosing prior to the procedure.
On the day of the procedure, both Dr. Kaufman and Dr. Dixon were in attendance. Monitored anesthesia care was provided to the patient as per department protocol. Three 7 Fr sheaths and an 8 Fr sheath were placed bilaterally in the femoral veins, in addition to a 6 Fr sheath in the right internal jugular and a 5 Fr sheath in the left femoral artery that would be utilized for beat-to-beat blood pressure monitoring. Three 6 Fr quadripolar pacing catheters were advanced to the high right atrium, His bundle, and right ventricular apex. A 5 Fr coronary sinus decapolar catheter was inserted through the right IJ into the coronary sinus for left atrial pacing and recording. After the catheters were placed, the patient developed his index focal atrial tachycardia. The tachycardia cycle length was 440 msec, the R-to-R interval ranged from 440 to 660 msec, the PR interval was demonstrating Wenckebach rhythm, the QRS interval was 80 msec, QT interval 280 msec, the AH interval varied from 180 msec to a Wenckebach cycle length, and the HV interval was 45 msec. Of interest, the earliest atrial depolarization was seen near coronary sinus pole 10-9. However, the His bundle electrogram was also very close to the earliest atrial electrogram. The surface EKG during tachycardia was suggestive of a tricuspid annular tachycardia, so we elected to first perform mapping in the right atrium.
Using the Carto 3 three-dimensional electroanatomical mapping system and the Biosense Webster MEM, we performed fast activation mapping of the entire right atrium. We took approximately 400 points with the PentaRay NAV catheter in less than 10 minutes. Review of these points demonstrated that the earliest atrial electrogram (when compared to CS poles 8-7) was seen at the lateral tricuspid annulus.
After a detailed map was made, a total of 16 applications of saline irrigated radiofrequency catheter ablation were delivered via the Biosense Webster ThermoCool SF DF curve ablating catheter. These applications ranged from 12 seconds to 120 seconds. The power delivered ranged from 30 watts to 45 watts, and these were delivered into 110–100 ohms impedance. Temperatures achieved were 30 to 35 ºC. This series of applications, delivered along the lateral tricuspid annulus, was successful in terminating the tachycardia, and there was no further inducible lateral wall tricuspid annular tachycardia.
Next, with pacing in the high right atrium, we were able to induce a different atrial tachycardia, which appeared to be emanating from the posterolateral tricuspid annulus, approximately 4 cm away from the site of the original tachycardia. Again, using the PentaRay NAV catheter, we remapped this tachycardia in the right atrium. The area of earliest atrial activation on this occasion was at the posterolateral tricuspid annulus, and atrial electrograms achieved at this site preceded the coronary sinus reference electrode by 87 msec. They also preceded the onset of the P-wave by 40 msec.
Three applications of saline irrigated energy were delivered using an SRO sheath for stability. These applications consisted of 40 and 41 watts delivered into 110 ohms impedance for periods of 120 seconds. These applications were successful in eliminating the second tachycardia (the second tachycardia cycle length was 600 msec prior to ablation).
A total of 19 applications of saline irrigated radiofrequency energy for a total delivery time of 22 minutes and 22 seconds were delivered. These applications were successful in eliminating all inducible atrial tachycardia.
At this point, programmed stimulation was performed in the drug-free state and during administration of isoproterenol. Single, double and triple extrastimuli coupled to atrial refractoriness could not induce atrial tachycardia post ablation. (It should be mentioned that the atrial pacing protocol was done in both the right and left atrium.) Single and double extrastimuli in the ventricle could not induce SVT post ablation.
At this point, the procedure was considered successful. Catheters and sheaths were removed and hemostasis was achieved. The patient was awakened from his sedation. There were no adverse events noted during the procedure. Recovery was uneventful, and the patient was discharged home after an appropriate recovery period.
Final impressions were focal atrial tachycardias emanating from the lateral tricuspid annulus and the inferolateral tricuspid annulus. A total of 19 applications of radiofrequency energy were delivered, all guided by three-dimensional electroanatomical mapping. Total fluoroscopy time utilized was 22 minutes and 16 seconds with 280 mGy — far below the reportable 2500 mGy. The reduced fluoroscopy time was attributed to the rapid mapping achieved with the PentaRay NAV catheter. Total procedure time (from stick to stop) was three hours and 38 minutes.
Conclusion
Our initial impression of this new technology was very favorable. The PentaRay NAV catheter facilitated an accurate and fast construction of the right atrial map. The initial map guided us to the tricuspid annulus and quickly identified it as the source of the arrhythmia. During this case, an additional atrial tachycardia was noted and two separate maps were needed. Previously, mapping of multiple atrial tachycardias was extremely time-consuming because of the need for multiple maps. The PentaRay NAV catheter allowed us to take 400 points in less than 10 minutes and formulate a single map, when other technology would have taken 45 to 60 minutes to achieve the same result. In summation, two atrial tachycardia maps of at least 400 points each were constructed in less than 20 minutes during this case.
Fluoroscopy and procedure times were decreased. These reductions resulted in decreased risk to the patient because he received less anesthesia and radiation. Costs were not increased but were indirectly decreased through the shortened procedure times. After the initial system configurations were accomplished, the interpretive learning curve for physicians and colleagues was vastly diminished. Catheter use was straightforward and uncomplicated. After reviewing the outcome of the case, physicians and colleagues alike agree that we met our objectives for the evaluation of this new adjunct to our care delivery system. Efficiency was positively impacted in a cost-effective manner while improving quality and patient safety.
Mark A. Dixon, DO and Scott Kaufman, DO are cardiac electrophysiologists in Northwest Indiana. Dr. Dixon is the Medical Director and Chris Atherton RN, BSN, MPA is the Director of the Arrhythmia Center at Indiana University Health La Porte Hospital, La Porte Indiana, and a part of Indiana University Health.
Disclosure: The authors have no conflicts or any relevant financial interests to disclose.











