ADVERTISEMENT
Axillary Access and Lateral Pocket Technique for Implantable Cardiac Devices
(Readers, please note the title of this article was changed; the original title was "Axial Access and Lateral Pocket Technique for Antiarrhythmic Device Placement.")
Introduction
Implantable pacemakers and implantable cardiac defibrillators (ICDs) have become increasingly prevalent treatments for various cardiac dysrhythmias. In the past five years, over 400,000 ICDs have been implanted annually in the United States alone.1 Through the years of implantation experience, several implant techniques have been adopted with varying clinical and cosmetic outcomes. The classic approach of modern medicine utilizes the subclavian vein as the point of access. This method, however, presents problematic issues such as subclavian crush, lead erosion, and subclavian stenosis.
Furthermore, patient cosmetic outcomes are typically ignored, despite many patients who consider  it to be more devastating than the disease process itself. The traditional technique leaves visible scars and a protruding device that can deter a patient’s recovery from a psycho-social aspect. The purpose of this paper is to introduce a modified axillary approach that has shown not only increased device longevity, but more importantly, improved cosmetic results.
it to be more devastating than the disease process itself. The traditional technique leaves visible scars and a protruding device that can deter a patient’s recovery from a psycho-social aspect. The purpose of this paper is to introduce a modified axillary approach that has shown not only increased device longevity, but more importantly, improved cosmetic results.
Modified Axillary Implantation Approach
The patient is first placed on the catheterization table in supine position with the arms adducted to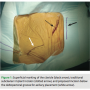 the body. The clavicular and shoulder areas are prepped laterally to the mid-axillary line. The anterior-axillary area, 2-3 cm below the deltopectoral groove, is then marked and local 1% lidocaine is applied along the track running to the subclavian vein (Figure 1).
the body. The clavicular and shoulder areas are prepped laterally to the mid-axillary line. The anterior-axillary area, 2-3 cm below the deltopectoral groove, is then marked and local 1% lidocaine is applied along the track running to the subclavian vein (Figure 1).
A 3 cm incision is made with a #10 blade at an oblique angle, lateral to the mid-clavicular line, so as to align with the natural axial skin folds (Figure 2). Cautery dissection is then performed through the subcutaneous layers in the medial direction down to the pectoral muscle level. The device pouch is created with blunt dissection between the muscular and 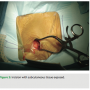 subcutaneous plains at the upper breast level (8-12 cm below the clavicle), in the mid-clavicular line.
subcutaneous plains at the upper breast level (8-12 cm below the clavicle), in the mid-clavicular line.
Venous access is obtained through single side puncture of the axillary vein during venogram through the peripheral IV access (Figure 3). A J-shaped guidewire is then passed through the needle (Figure 4), and standard sheath and lead placement follows. Typically 5 cm of lead length is added compared to standard implant due to a more lateral approach.
Once the endocardial leads are secured, the leads are tested for pacing and sensing thresholds.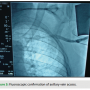 The device is connected to the generator and placed into the pocket medially while a single anchoring suture is used to secure the device and prevent lateral migration (Figure 5). Antibiotic flush and standard closure procedures are used (Figures 6-8).
The device is connected to the generator and placed into the pocket medially while a single anchoring suture is used to secure the device and prevent lateral migration (Figure 5). Antibiotic flush and standard closure procedures are used (Figures 6-8).
Discussion
With our proposed approach to device implantation, we are able to gain several advantages. Our method allows for a truncated procedure and fluoroscopy time. We recorded an average procedure time of 42 ± 11 min and a fluoroscopy time of 3.2 ± 2.1 min. In comparison, implantation via the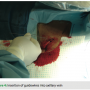 traditional method requires a procedure time of 76 ± 22 min total with a fluoroscopy time of 7.5 ± 5.2 min.2
traditional method requires a procedure time of 76 ± 22 min total with a fluoroscopy time of 7.5 ± 5.2 min.2
In addition, entering through the axillary vein rather than the subclavian spares the proximal part of the vein for future re-entry if necessary (e.g., device upgrade). Moreover, visualization of the vein during needle introduction allows for direct puncture into the vein, avoiding trauma to the posterior and avoiding the risk of pneumothorax. While previously published complication rates suggest a 1% risk of pneumothorax through subclavian access,2-5 our single-center experience has had zero cases of pneumothorax. Placing the device in a different position further enables us to avoid clavicular crushing of the lead wires, resulting in increased lead longevity. Another important advantage is that the generator placement may also lower defibrillation threshold as the can is an active defibrillation pole, and the defibrillation energy delivery is closer to the heart (Table 1).
wires, resulting in increased lead longevity. Another important advantage is that the generator placement may also lower defibrillation threshold as the can is an active defibrillation pole, and the defibrillation energy delivery is closer to the heart (Table 1).
The largest difference, however, is the aesthetic outcome. Often ignored by the traditional approach is the psychosocial impact post implantation. For patients, acceptance can relate directly to cosmetic outcome as a protruding device can become a daily visual reminder of their condition (Figure 9). They may find themselves facing several psychological barriers in the recovery process, including body image concerns, which may manifest into anxiety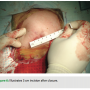 or depression. Confronted with these issues, patients may begin withdrawing from society, leading to a reduced quality of life.6 Patients struggling to overcome these obstacles may find that a conspicuous scar and a device jutting from their chest only serve as a daily reminder of their condition. Our implantation technique addresses this handicap to patient recovery in a manner that previous methods have failed to do.
or depression. Confronted with these issues, patients may begin withdrawing from society, leading to a reduced quality of life.6 Patients struggling to overcome these obstacles may find that a conspicuous scar and a device jutting from their chest only serve as a daily reminder of their condition. Our implantation technique addresses this handicap to patient recovery in a manner that previous methods have failed to do.
By moving the device to a fleshier region and making an incision in the natural skin folds, we are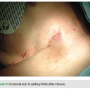 able to conceal the device and scar. After fully healing, this leaves the presence of the foreign object all but invisible (Figures 10 and 11). This lateral placement also effectively diminishes contact with common irritants that can aggravate the pocket site such as a bra strap or seat belt, decreasing the possibility of tissue erosion (Figure 12). Ultimately, this leads to better healing and a more comfortable recovery.
able to conceal the device and scar. After fully healing, this leaves the presence of the foreign object all but invisible (Figures 10 and 11). This lateral placement also effectively diminishes contact with common irritants that can aggravate the pocket site such as a bra strap or seat belt, decreasing the possibility of tissue erosion (Figure 12). Ultimately, this leads to better healing and a more comfortable recovery.
Positive feedback from our patients has affirmed that our method of device implantation has provided for a smoother transition back to the wide variety of activities that our patients enjoy. At our “Heart to Heart Talk” support group, patients shared with us their post-implantation device experience:
provided for a smoother transition back to the wide variety of activities that our patients enjoy. At our “Heart to Heart Talk” support group, patients shared with us their post-implantation device experience:
Female, 48, history of sudden cardiac arrest 2 weeks post implant: “I have forgotten that it is there. I feel protected, and live my life to the fullest.”
Male, 47, triathlete, passionate cyclist who does 100-mile races, and competes against 20 year olds: “[I] Feel good, feel protected, and love to ride.”
Female, 45, mother, martial arts professional: “I can still wear sleeveless tops and low-cut blouses, and I can still kick butt.”
and I can still kick butt.”
A petite female, 75, active ballroom dancer, with a history of atrial fibrillation and heart failure: “I love ballroom dancing and wearing beautiful gowns.”
A newlywed male, 25, history of hypertrophic obstructive cardiomyopathy: “I am not embarrassed to take off my shirt.”
Conclusions
As the spotlight in modern medicine has grown to include the psychological as well as the physical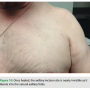 well-being of ICD patients, concerns about cosmetic outcomes of device implantation have come to light. With our modified technique that creates a more lateral device pocket and an incision along the axial vein, we provide an alternate approach that addresses the concerns of many patients. It fuses improved technical results with enhanced cosmetic benefit. The significance of aesthetic value in patient acceptance and coping of the foreign device as well as recovery back to their normal lives cannot be understated.
well-being of ICD patients, concerns about cosmetic outcomes of device implantation have come to light. With our modified technique that creates a more lateral device pocket and an incision along the axial vein, we provide an alternate approach that addresses the concerns of many patients. It fuses improved technical results with enhanced cosmetic benefit. The significance of aesthetic value in patient acceptance and coping of the foreign device as well as recovery back to their normal lives cannot be understated.
Disclosures: Dr. Whittington, Ms. Huang, Ms. Olsen, Ms. Kusne, and Ms. Dacanay have no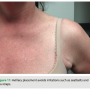 conflicts of interest to report. Outside the submitted work, Dr. Su reports consultancy with Medtronic, honoraria to BIOTRONIK, and grants/grants pending to his institution from Medtronic.
conflicts of interest to report. Outside the submitted work, Dr. Su reports consultancy with Medtronic, honoraria to BIOTRONIK, and grants/grants pending to his institution from Medtronic.
References
- Buch E, Boyle N, Belott P. Pacemaker and defibrillator lead extraction. Circulation. 2011;123:e378-e380.
- van Rugge FP, Savalle L, Schalij M. Subcutaneous single-incision implantation of
 cardioverter-defibrillators under local anesthesia by electrophysiologists in the electrophysiology laboratory. Am J Cardiol. 1998;81:302-305.
cardioverter-defibrillators under local anesthesia by electrophysiologists in the electrophysiology laboratory. Am J Cardiol. 1998;81:302-305. - Jordaens L, Vertongen P, Provenier F, et al. A new transvenous internal cardioverter-defibrillator: implantation technique, complications, and short-term follow-up. Am Heart J. 1995;129:251-258.
- Romeyer-Bouchard C, Da Costa A, Abdellaoui L, et al. Simplified cardiac resynchronization implantation technique involving right access and a triple-guide/single introducer approach. Heart Rhythm. 2005;2:714-719.
- Pavia S, Wilkoff B. The management of surgical complications of pacemaker and
 implantable cardioverter-defibrillators. Curr Opin Cardiol. 2001;16:66-71.
implantable cardioverter-defibrillators. Curr Opin Cardiol. 2001;16:66-71. - Hazelton G, Sears S, Kirian K, Matchett M, Shea J. Cardiology Patient Page. Coping with my partner’s ICD and cardiac disease. Circulation. 2009;120:e73-e76











